Regulators are attacking ongoing drug shortages, and Canada is an example worth examining. In November 2022, Health Canada created a Drug Shortages Task Force. Canadians and other stakeholders are invited to weigh in during the public consultation period – which ends August 4, 2023 – regarding how best to improve the availability of drugs and health products.
Why does this matter outside Canada? Once adopted, medical and healthcare legislation tends to move quickly from country to country. We are following this legislation closely because regulatory pressures tend to migrate in a generally harmonized manner.
Drug shortages have heightened
The COVID-19 pandemic created a record surge in demand due to the large numbers of critically ill patients. This increase in demand strained a highly complex global supply chain and raised awareness of issues related to the stability and security of medical supplies.
While the Department of Health and Human Services (HHS) in the U.S. declared the end of the Public Health Emergency (PHE) for COVID-19, on May 11, 2023, the challenges of drug shortages continue to rise. A recent tornado that ripped off the roof of a Pfizer plant is a reminder of just how fragile pharmaceutical supply chains still are. It’s being reported that the Pfizer factory makes nearly 25% of all sterile injectable medicines used in U.S. hospitals.
“According to the American Society of Health-System Pharmacists (ASHP), the U.S. health care system currently is experiencing the most drug shortages since 2014,” Stacey Hughes of the American Hospital Association said in a letter to the senate. “The number of active drug shortages in the U.S. reached a new peak of 301 in the first quarter of 2023.”
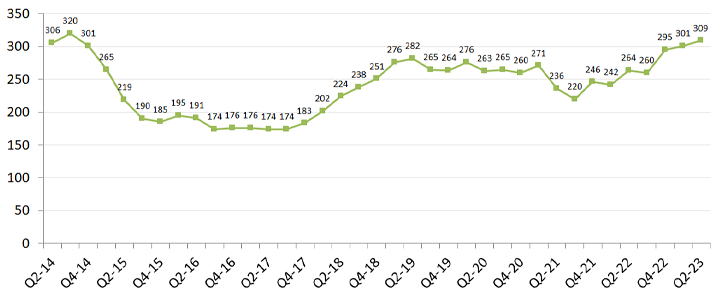
Figure 1: National drug shortages by quarter, 10-year trend. Each points represents number of active shortages at the end of each quarter (source: University of Utah Drug Information Service).
Drug shortages can result in a dramatic negative affect on overall patient care. A shortage of proper medication can cause delays in treatment, increase the risk of medication errors, and sometimes result in the use of less effective alternative treatments. Given the sensitivity and complexity of our healthcare systems, it is not just drug manufacturers that are impacted. Additional groups that care deeply about the resilience of our healthcare supply chain include group purchasing organizations (GPOs), wholesalers, distributors, pharmacies, hospitals clinics, health care professionals and their respective associations and most importantly – patients.
Worldwide public concerns
The concerns surrounding drug shortages and the efficacy of our broader healthcare systems go beyond the four walls of regulatory authorities – the broader public has also begun to express concerns. According to Health Canada’s website, “Recent public opinion research shows that 56% of the population is concerned about drug shortages.” History has shown that public concerns tend to drive political pressure. Hence, the demand for solutions that help ensure the resiliency of our healthcare supply chain can be expected to increase for the foreseeable future.
“Hospitals and health systems have long been concerned about chronic and increasing drug shortages that have serious consequences for patient safety, quality of care and access to therapies. Addressing drug shortages is complex and costly to hospitals and health systems in terms of staff time and other resources required to manage the shortages.”
Canada isn’t the only country responding to these concerns. Regulatory authorities around the globe are exploring ways in which to help improve the stability, visibility, and resilience of pharmaceutical and broader healthcare supply chains.
Health Canada’s four focus areas
Health Canada’s website proposes four key areas for action:
- Improved communication and transparency
- Agile regulatory toolbox
- Greater supply chain visibility
- Enhanced response to supply and demand
This legislation was designed to address a global problem that Health Canada calls out explicitly: “We’re seeing an increase in the number of critical national shortages, and shortages are lasting longer.”
Reporting will be a large component of this emerging legislation. Health Canada has mandated drug shortage reporting since March 2017. And, even prior to this a Multi-Stakeholder Steering Committee on Drug Shortages (MSSC) was established in 2012.
Furthering these prior efforts, on November 27, 2020, “The Minister of Health made the Interim Order respecting drug shortages (safeguarding the drug supply). This interim order allowed Health Canada to compel a marketing authorization holder (MAH) or drug establishment license (DEL) holder to provide information on actual or anticipated drug shortages. The provisions have been transitioned to permanent provisions in the FDR, which took effect on November 28, 2021.”
It’s important to note that this legislation could broaden to apply to other industries.
Regulatory authorities around the globe are implementing policies for improved visibility and mandatory notification related to incidents in the pharmaceutical supply chain. Health Canada specifically states that there is a: “…need to take additional action to build resilience in the supply chain for Canada’s drugs, medical devices, and life-sustaining foods.”
How AI can help supply chains prepare
How are drug manufacturers and those in the broader pharmaceutical supply chain responding to these new challenges and demands for supply chain stability, visibility, and resiliency? Supply Chain Risk Management Centers of Excellence are being formed. Those involved in the pharmaceutical supply chain are implementing software solutions that enable broader visibility and sub-tier mapping of suppliers of critical components needed to support continuity of supply of medicinal products. Supply chain concentration risks are being proactively identified and risk-mitigation plans are being implemented to help thwart issues before they impact patient outcomes.
Furthermore, supply chain risk and incident management solutions are being used by drug manufacturers, GPOs, drug distributors, and others in the broader healthcare supply chain to increase awareness of relevant and timely impacts to nodes in their up-stream supply chain.
Health Canada’s reporting requirements around anticipated shortages increases the importance of the use of Supply Chain Risk Management (SCRM) solutions. If a manufacturer believes a shortage is “likely to occur,” the notification must be made “within five calendar days of becoming aware of an anticipated shortage/discontinuation that is expected to happen in the next six months” and “within five calendar days of becoming aware of an actual shortage.” There is also further required notification within two calendar days of becoming aware of any “status change.”
One of the most effective ways to meet these requirements is through the implementation of sophisticated software tools that enable sub-tier mapping of a highly complex supply chain. New solutions on the market are leveraging AI and ML technology to map sub-tier supply chains more efficiently.
Everstream Analytics aligns well to Health Canada’s four focus areas by providing an AI-powered platform and features to meet the needs of organizations looking to comply. Everstream’s platform connects to each focus area as follows:
Improved communication and transparency. Everstream Reveal offers real-time monitoring and insights to help quickly mitigate risk. Internal communication and collaboration with suppliers is just an easy click away as events occur.
Agile regulatory toolbox. Everstream Reveal monitors for real-time updates on regulatory changes that impact global supply chains. This increases agility and responsiveness.
Greater supply chain visibility. Everstream Discover automatically “connects-the-dots” in the multi-tier supply chain, creating the enhanced visibility required to meet the changes in today’s business climate.
Enhanced response to supply and demand. Everstream Reveal is constantly monitoring for global incidents that can cause impacts to supply and demand. Enabling rapid response to balance inventory and supply sources as the need arises.
Get the update on quality issues, regulatory shifts, insolvencies, and other growing risks for hospitals, healthcare providers, and GPOs.